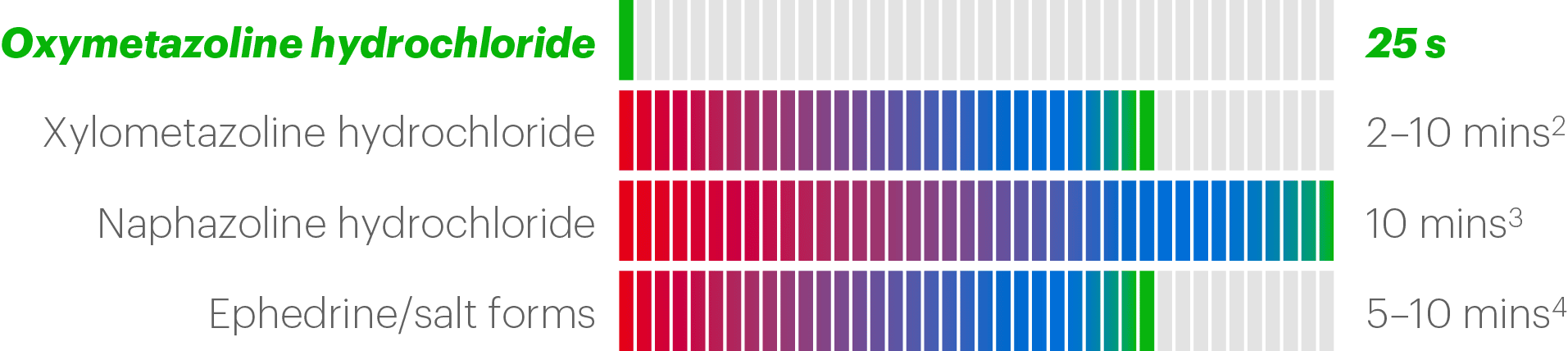
Clarispray® begins to work after just 25 seconds1
while other nasal decongestants take a few minutes to work.

Oxymetazoline has been clinically proven to work significantly faster than placebo* — 25s vs 90s (p<0.001).
References:
1.Reinecke S, Tschaikin M. MMW Prog Med Original 2005;147:113–18. 2.Graf C et al. Int J Gen Med 2018;11:275–83. 3.Naphazoline (OTC). Medscape Drugs and diseases. Available from: https://reference.medscape.com/drug/- privine-naphazoline-343407#10. Last accessed May 2024. 4.Trakarnsilpa C et al. J Med Assoc Thai 2017;100:358–64.
Clarispray® increases the area of the nasal passages 2.3-fold
In a study looking at resistance and airflow in subjects with and without nasal airway obstruction (NAO).1
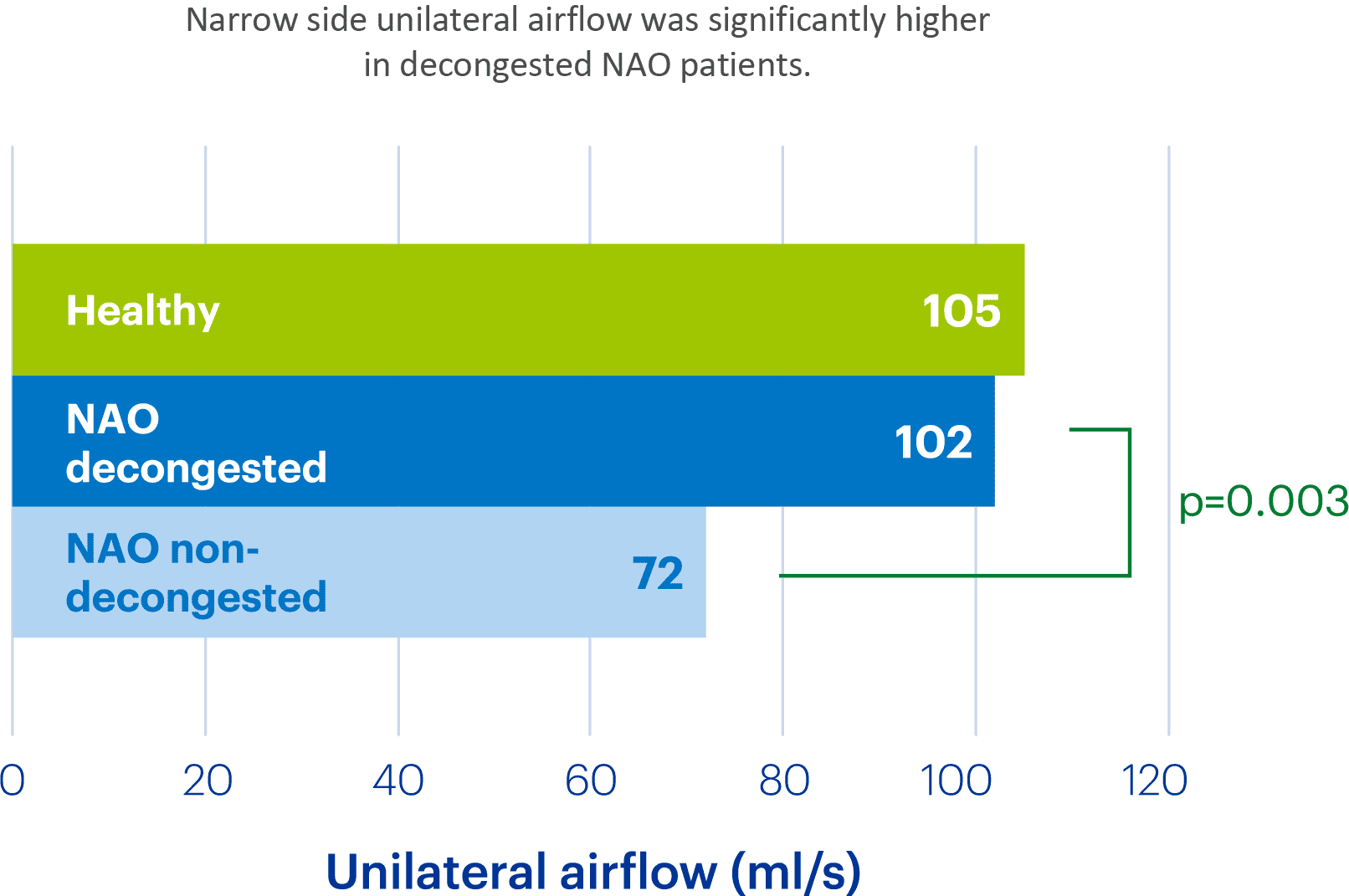
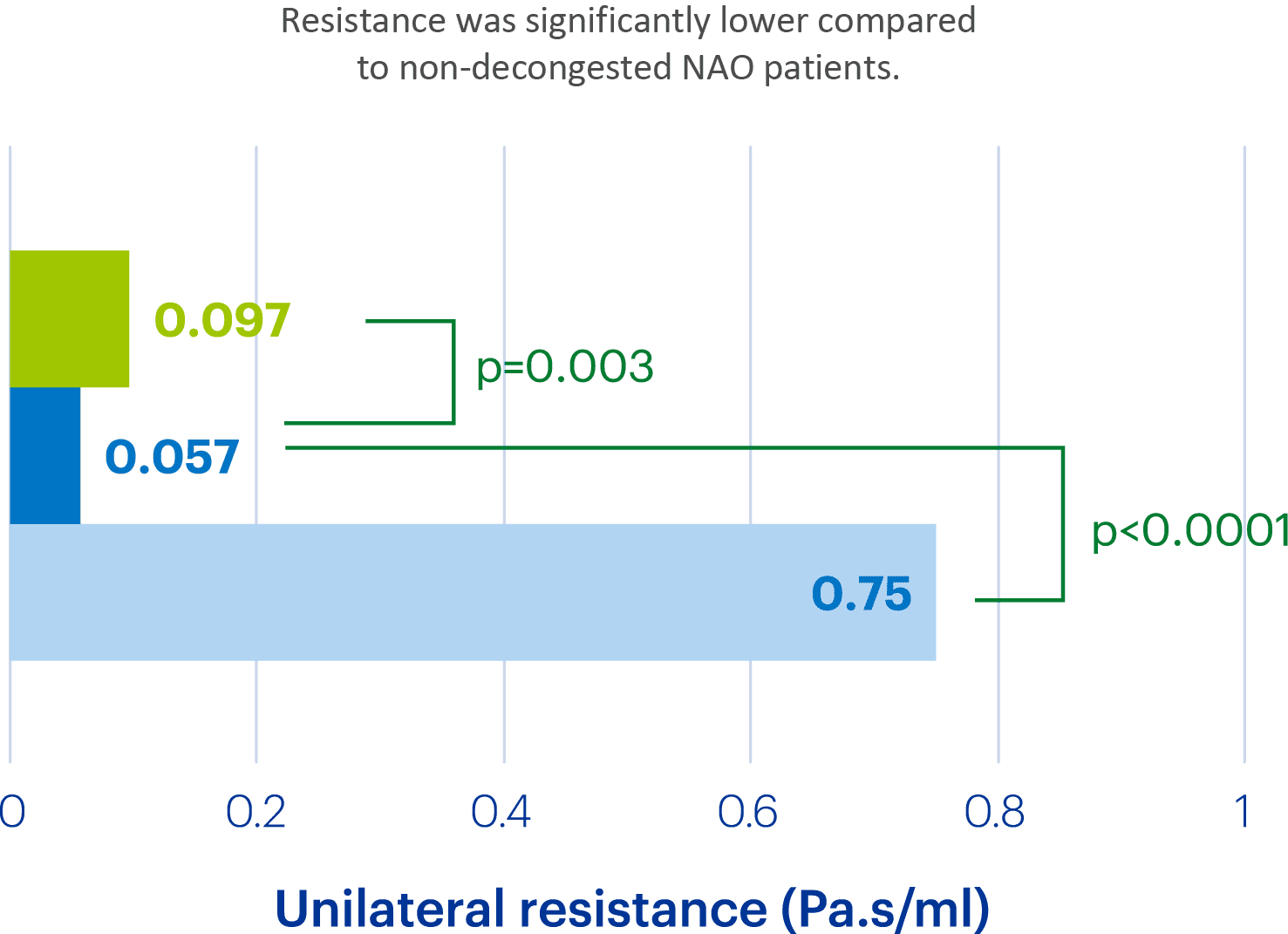
1.Hamdan AT et al. Otolaryngol Head Neck Surg 2024;170:1696–704.

Long-lasting relief
One dose of ClariSpray® provides significant congestion relief for 12 hours.1
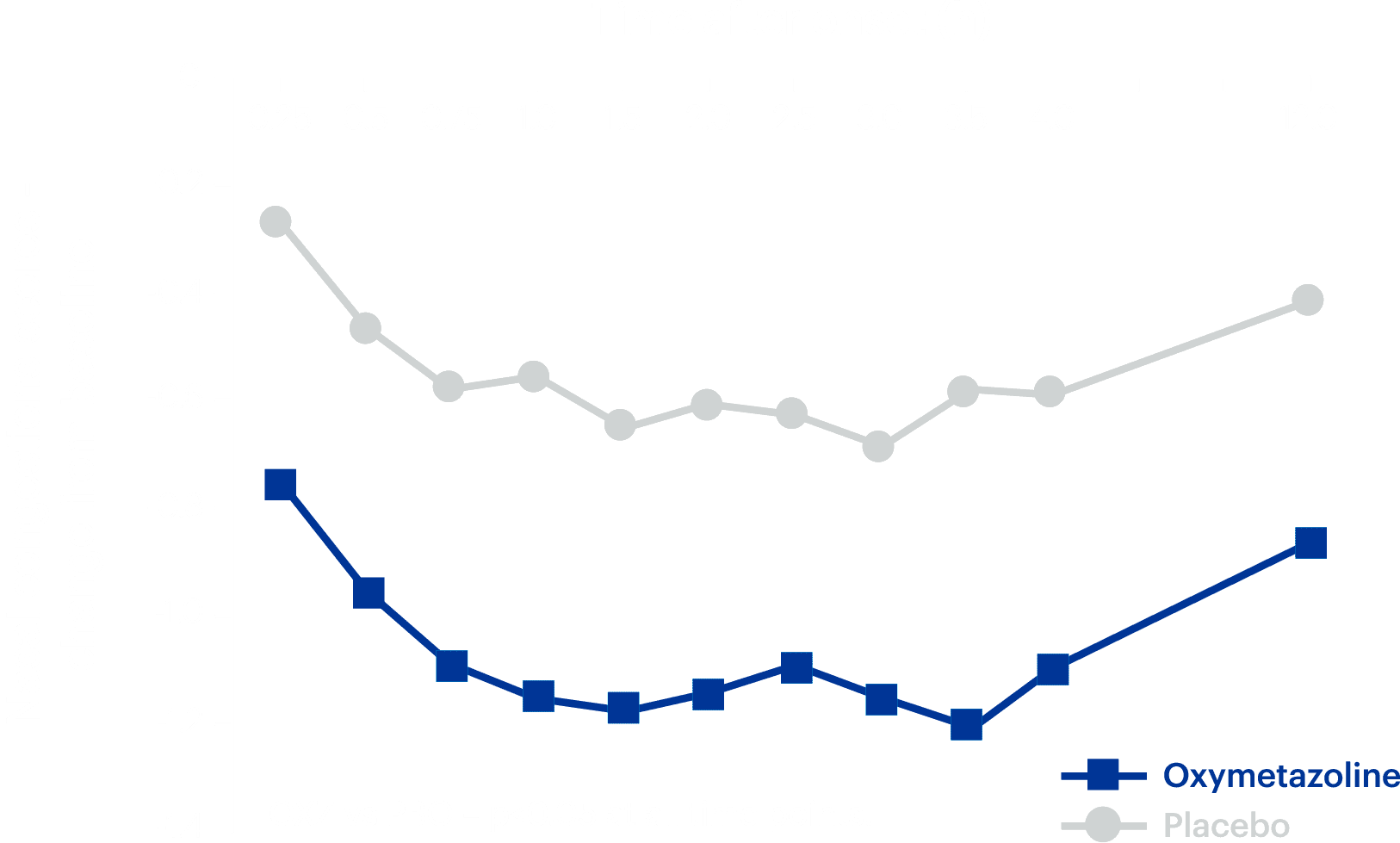
1. Meltzer EO et al. Am J Rhinol Allergy 2013;27:102–8.
Clarispray® has a low risk of adverse events
- Oxymetazoline, the active ingredient in ClariSpray®, is generally well tolerated when used as directed.1
- As a topical therapy it is possible to deliver directly to the receptor site at the source of congestion with a reduced risk of systemic side effects compared to oral therapies.2
- In clinical trials, it was associated with a low incidence of treatment-related adverse events.3
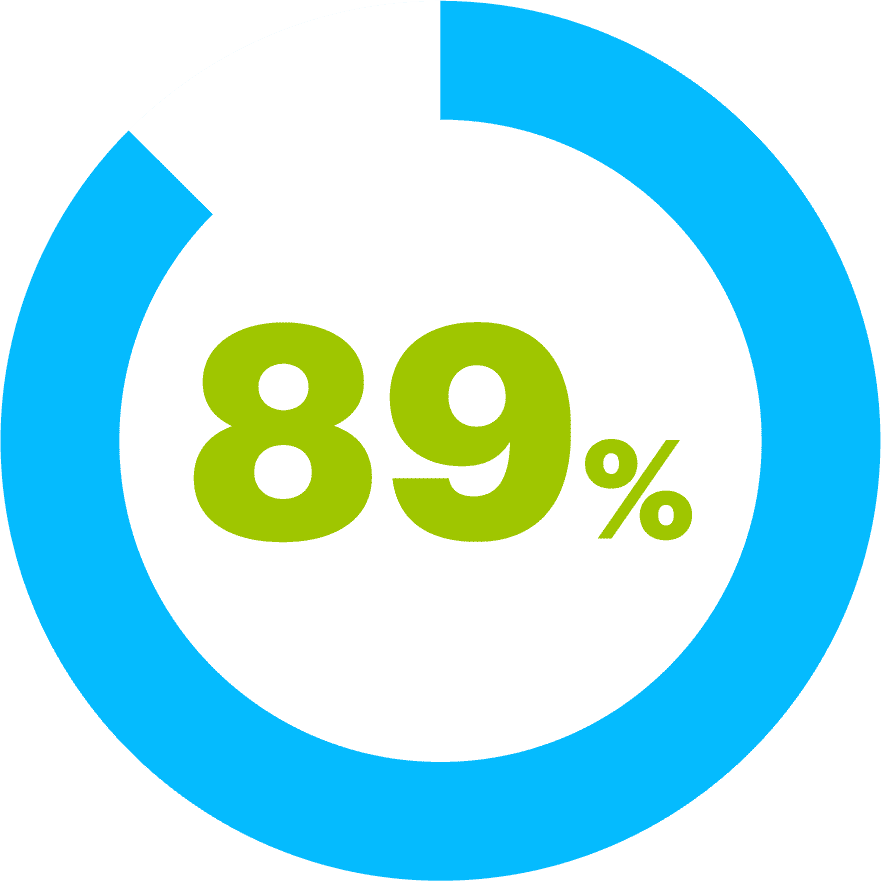
of users stated that ClariSpray® was pleasant to use.4
1.Krouse JH. In: Managing the Allergic Patient. Elsevier 2008. 2.Watts AM et al. Front Pharmacol 2019;10:294. 3.Meltzer EO et al. Am J Rhinol Allergy 2013;27:102–8. 4.Bayer. Data on file.
Clarispray® no-drip technology rates highly with users
In a post-marketing study of 101 patients* experiencing mild, moderate, and severe congestion:1
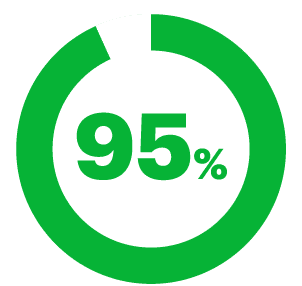
Agreed that ClariSpray® stayed in their nose.
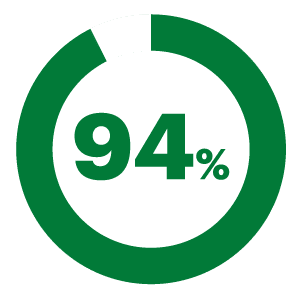
Found the nasal spray experience comfortable.
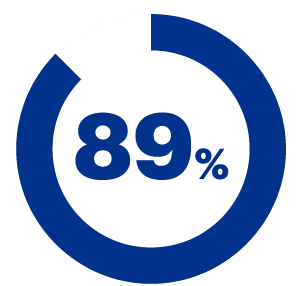
Agreed that the product was pleasant to use.
*Results from a market research study conducted in the USA to evaluate over-the-counter consumer attitudes and uses of nasal sprays.
USA: United States of America.
Reference:
1. Bayer, data on file.
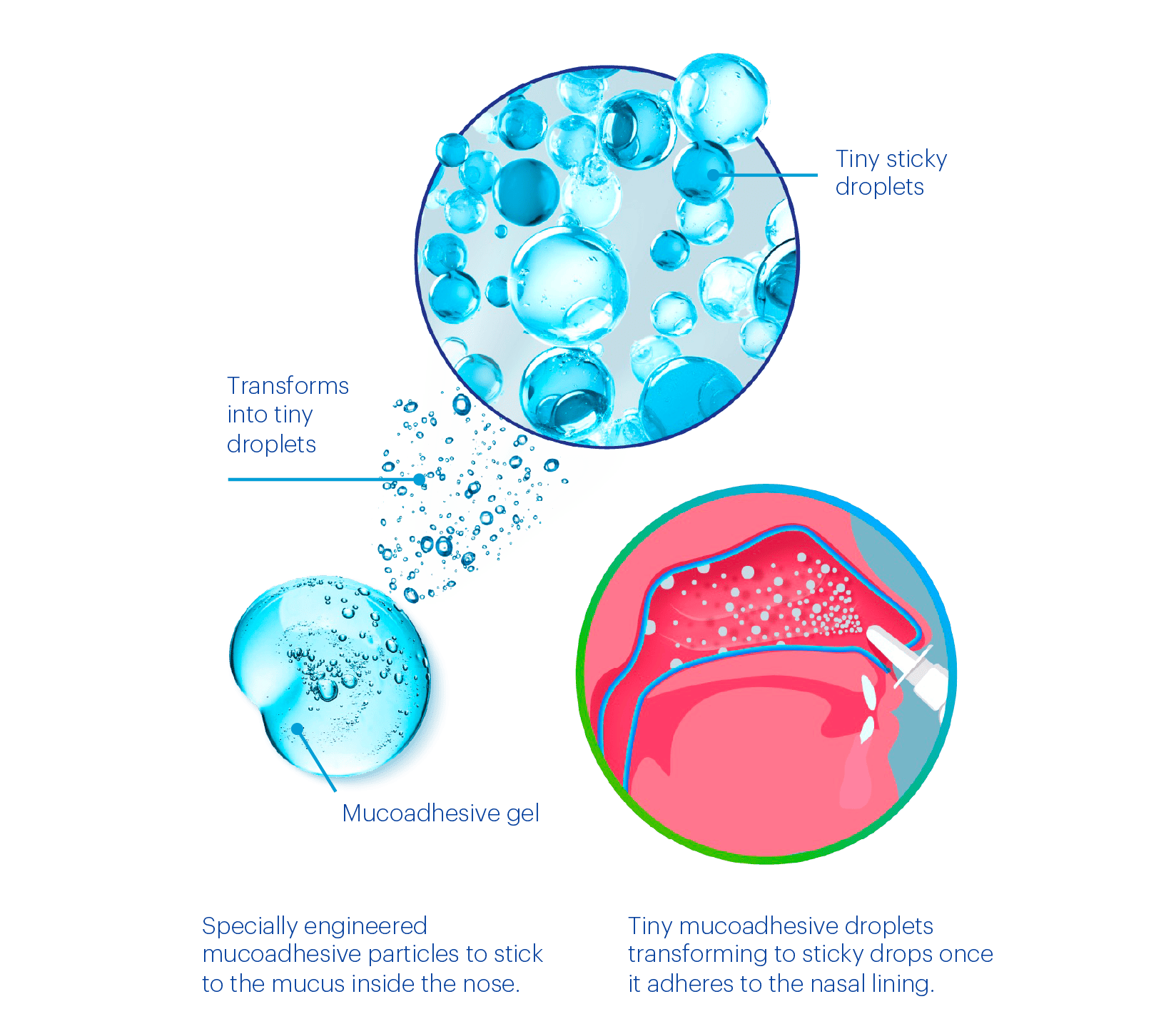
Clarispray® has a unique, innovative no-drip technology
- ClariSpray® has a thixotropic formulation that transforms the mucoadhesive gel into tiny sticky droplets when shaken.1
- Once sprayed inside the nose, the droplets adhere to the nasal mucosa.1
- The solution then reverts to its gel-like state and remains on the nasal mucosa.1
1. Bayer. ClariSpray Original and Menthol SMPC.
No other nasal decongestant spray relieves congestion for longer than ClariSpray®
- Suitable for adults, adolescents, and children aged 6–10 (with adult supervision).1
- Can be taken for up to 7 days. If symptoms persist, an interval of several days should elapse before treatment is restarted.1
- Can be used in pregnancy if used as recommended.*1
Recommended frequency of usage
every 12 hours.1
*Caution should be exercised in patients with hypertension or signs of reduced placental perfusion.
Frequent or prolonged use of high doses may reduce placental perfusion.
Reference:
1. Bayer. ClariSpray Patient Information Leaflet. Middle East.

Clarispray® For Instant, Powerful, Long-Lasting
relief from nasal congestion

Relief in just 25 seconds1, lasting up to12 hours.2

Unique no-drip technology — stays where it’s sprayed, so no unpleasant dripping or bitter taste.3

Does not cause drowsiness.4
1. Reinecke S, Tschaikin M. MMW Prog Med Original 2005;147:113–18.
2. Meltzer EO et al. Am J Rhinol Allergy 2013;27:102–8.
3. Bayer, ClariSpray Original and Menthol SMPC.
4. Bayer, ClariSpray Patient Information Leaflet. Middle East.