Efficacy

The Majority of Pediatric Patients respond well to Claritine™1
82%
of children treated with Claritine™ (loratadine) had a good or excellent therapeutic response1
1. Lutsky BN, Kl.se P, Melon J, et al. A comparative study of the efficacy and safety of loratadine syrup and terfenadine suspension in the treatment of 3- to 6-year-old children with seasonal allergic rhinitis. Clin Ther. 1993;15(5):855-65Claritine™ is effective across multiple allergic rhinitis symptoms in children1
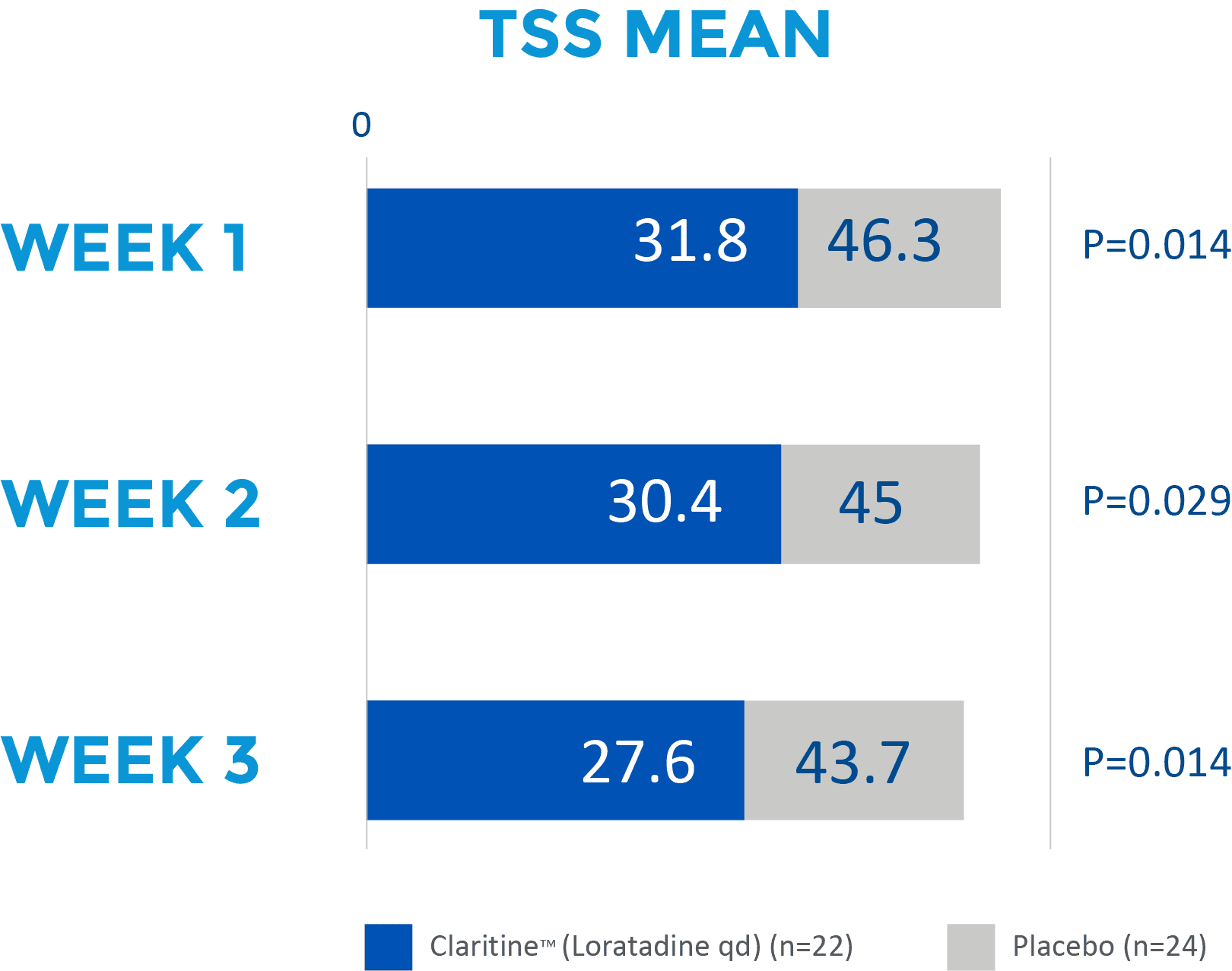
Children receiving Claritine™ had a lower TSS across multiple symptoms when compared with a placebo1
Adapted from Yang YH et al. 2001Results from a 3-week, double-blind, placebo-controlled, randomised trial involving 46 children (aged 3−12 years) with allergic rhinitis.2
TSS=total symptom score; qd=once daily.
The efficacy of loratadine syrup for these 4 symptoms was evaluated by comparing the last week (day 15-day 21) symptom scores from the diary cards between the two groups
1. Yang YH, Lin YT, Lu MY, et al. A double-blind, placebo-controlled, and randomized study of loratadine (Claritine™) syrup for the treatment of allergic rhinitis in children aged 3 to 12 years. Asian Pac J Allergy Immunol. 2001;19(3):171-55
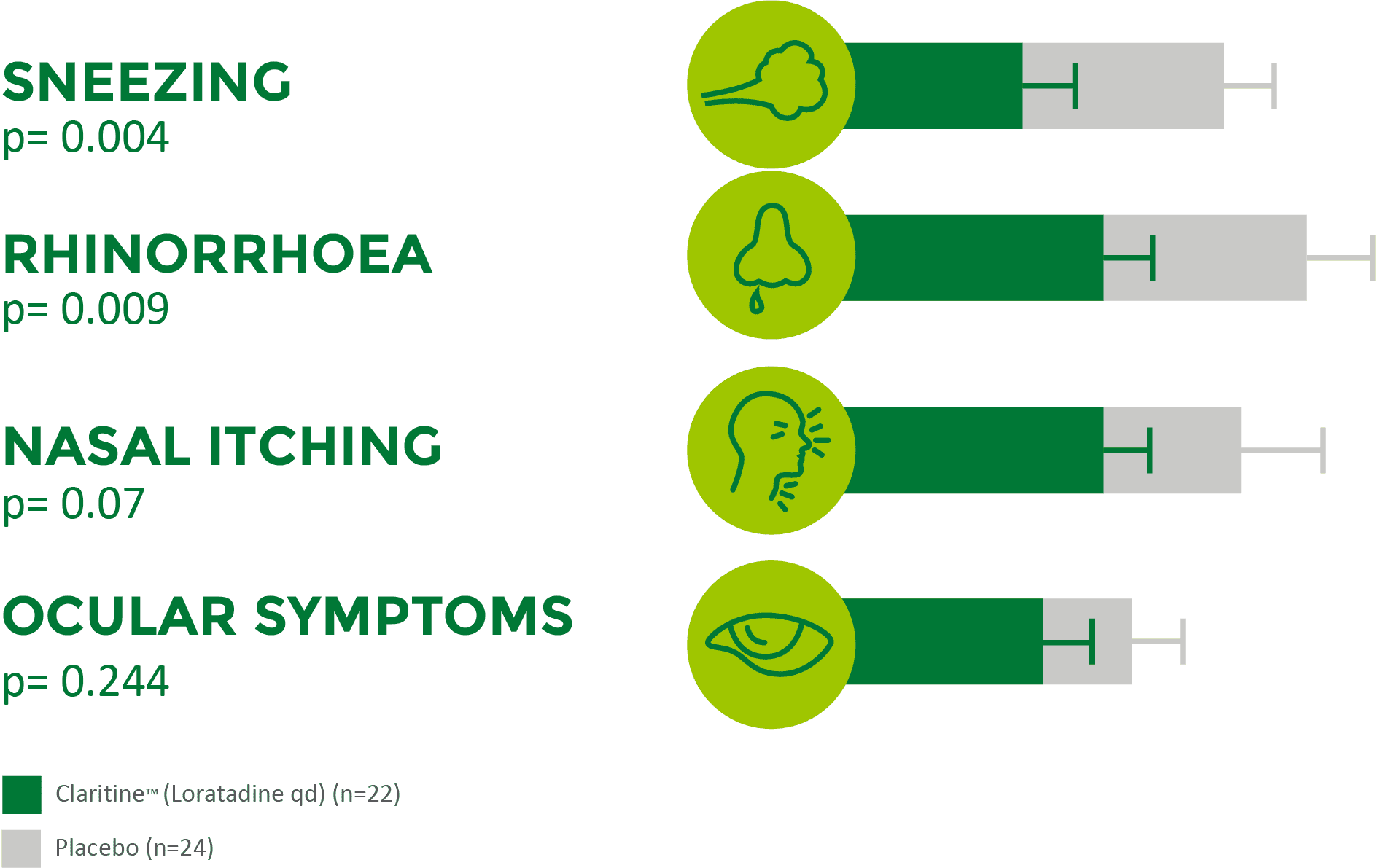
Claritine™ is effective across multiple allergic rhinitis symptoms in children1
Children receiving Claritine™ (loratadine qd) had a significantly lower TSS vs placebo during the 3-week therapeutic period1
Adapted from Yang YH et al. 2001Results from a 3-week, double-blind, placebo-controlled, randomised trial involving 46 children (aged 3−12 years) with allergic rhinitis.2
TSS=total symptom score; qd=once daily.
The efficacy of loratadine syrup for these 4 symptoms was evaluated by comparing the last week (day 15-day 21) symptom scores from the diary cards between the two groups
1. Yang YH, Lin YT, Lu MY, et al. A double-blind, placebo-controlled, and randomized study of loratadine (Claritine™) syrup for the treatment of allergic rhinitis in children aged 3 to 12 years. Asian Pac J Allergy Immunol. 2001;19(3):171-55
With one dose per day that lasts for 24 hrs, Claritine™ is the shield that allows children to treat their allergy monster all day and night1,2
1. Mendoza de Morales T, S.nchez F. Clinical efficacy and safety of a combined Loratadine betamethasone oral solution in the treatment of severe pediatric perennial allergic rhinitis. World Allergy Organ J. 2009;2(4):49-53 2. Yang YH, Lin YT, Lu MY, et al. A double-blind, placebo-controlled, and randomized study of loratadine (Claritine™) syrup for the treatment of allergic rhinitis in children aged 3 to 12 years. Asian Pac J Allergy Immunol. 2001;19(3):171-5
Tolerability
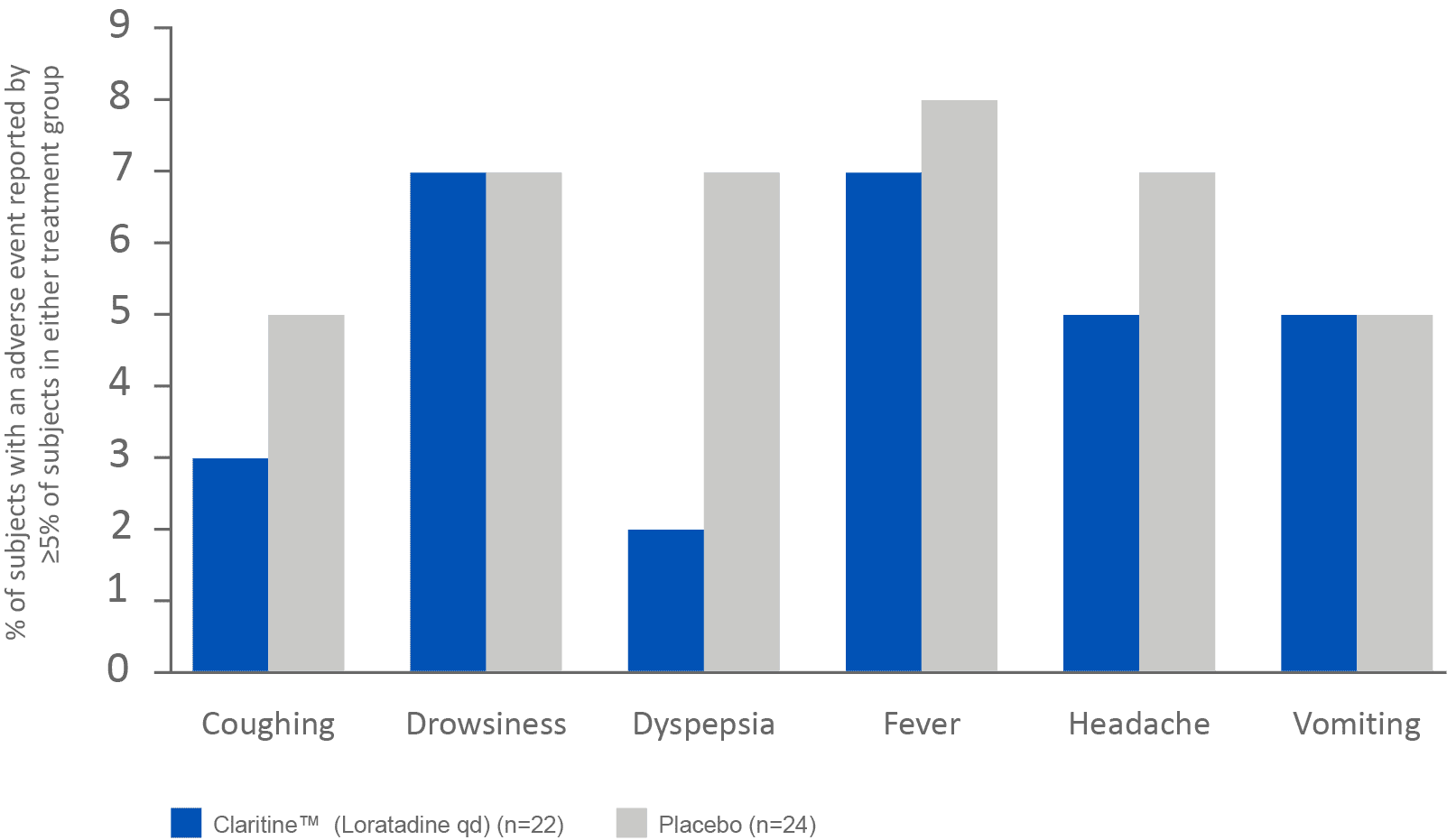
Claritine™ is well tolerated in the treatment of AR in patients as young as 2 years old1
qd=once-dailyAdapted from Salmun LM et al. 2000.
Results from a 15-day randomised, double-blind placebo controlled study evaluating the safety of Claritine™ (loratadine 5 mg qd) in 121 paediatric AR patients between 2 and 5 years of age.1
1. Salmun LM, Herron JM, Banfield C, et al. The pharmacokinetics, electrocardiographic effects, and tolerability of loratadine syrup in children aged 2 to 5 years. Clin Ther. 2000;22(5):613-21.
Claritine™ (loratadine 5 mg qd) tolerability study in paediatric patients between 2 and 5 years of age1
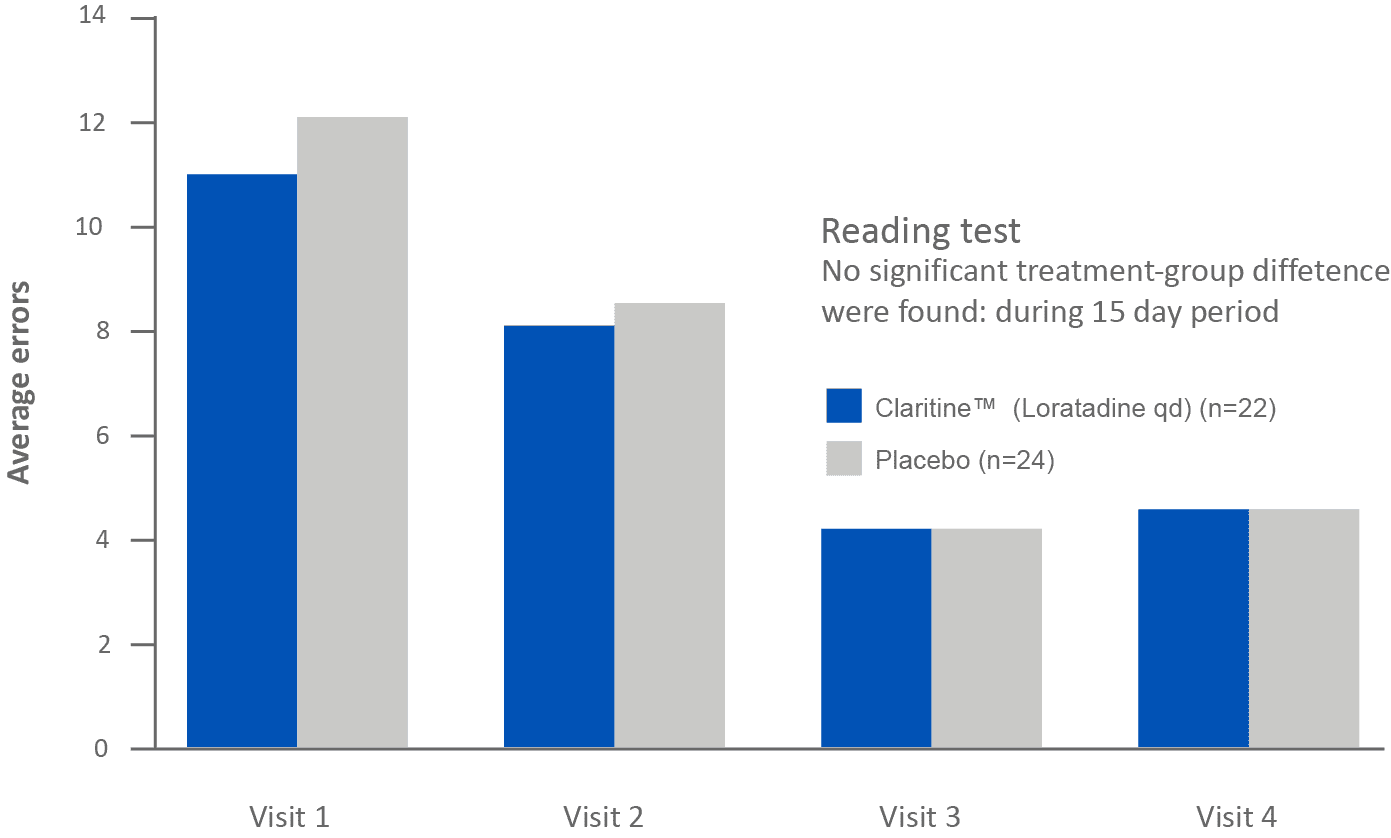
Short-term administration of Claritine™ (loratadine) does not impair children’s performance in school1
Adapted from Bender et al. 2001. Results from a parallel-group, double-blind, placebo Controlled study on 63 children between 8 and 10 years of age with allergic rhinitis.1. Bender BG, McCormick DR, Milgrom H. Children’s school performance is not impaired by short-term administration of diphenhydramine or loratadine. J Pediatr. 2001;138(5):656-60
Children’s learning performance is not significantly affected by Claritine™ (loratadine 10mg) at school1
Dosing
Children aged 2 to 12 years are
dosed according to their weight:
Body weight above 30 kg:
Take once daily 10 ml (measuring cup filled to 10 ml line).
Body weight 30 kg or less:
Give once daily 5 ml (measuring cup filled to 5 ml line).
1. Claritine Abbreviated Prescribing Information

Summary
-

Claritine™ offers 24-hour relief from allergic rhinitis symptoms triggered by indoor and outdoor allergens.1,2
1-Yang YH, Lin YT, Lu MY, et al. A double-blind, placebo-controlled, and randomized study of loratadine (Claritine™) syrup for the treatment of allergic rhinitis in children aged 3 to 12 years. Asian Pac J Allergy Immunol. 2001;19(3):171-5 2- M.sges R, Klimek L. Today’s allergic rhinitis patients are different: new factors that may play a role. Allergy. 2007 Sep;62(9):969-75. -

Claritine™ is administered once a day.1,2
1. Yang YH, Lin YT, Lu MY, et al. A double-blind, placebo-controlled, and randomized study of loratadine (Claritine™) syrup for the treatment of allergic rhinitis in children aged 3 to 12 years. Asian Pac J Allergy Immunol. 2001;19(3):171-5 2. Kay GG, Harris AG. Loratadine: a non-sedating antihistamine. Review of its effects on cognition, psychomotor performance, mood and sedation. Clin Exp Allergy. 1999;29 Suppl 3:147-50 -

As Claritine™ is a non-drowsy antihistamine, it has no significant impact on a child’s learning activities.1,2
1.Mir E, Panjabi C, Shah A. Impact of allergic rhinitis in school going children. Asia Pac Allergy. 2012;2(2):93–100. 2. Bender BG, McCormick DR, Milgrom H. Children’s school performance is not impaired by short-term administration of diphenhydramine or loratadine. J Pediatr. 2001;138(5):656-60 -

Claritine™ has a favorable safety profile and is well tolerated by children.1,2
1. M.sges R, Klimek L. Today’s allergic rhinitis patients are different: new factors that may play a role. Allergy. 2007 Sep;62(9):969-75 2. Salmun LM, Herron JM, Banfield C, et al. The pharmacokinetics, electrocardiographic effects, and tolerability of loratadine syrup in children aged 2 to 5 years. Clin Ther. 2000;22(5):613-21 -

In liquid and tablets formulations, Claritine™ is easy to administer and facilitates adherence.1,2
1.Venables R, Batchelor H, Hodson J et al. Determination of formulation factors that affect oral medicines acceptability in a domiciliary paediatric population. Int J Pharm. 2015;480(1-2):55-62 2. Davies EH, Tuleu C. Medicines for children: a matter of taste. J Pediatr. 2008;153(5):599-604, 604.e1-2

